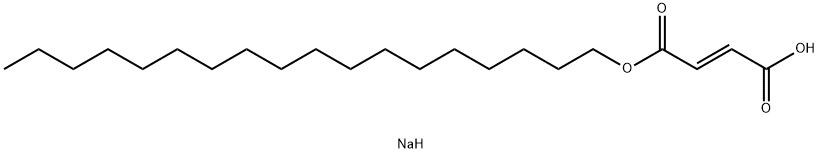
Sodium Stearyl Fumarate:General description,Application,and Production
Sodium Stearyl Fumarate is one type of lubricant. It was introduced as a suitable lubricant for tableting. Sodium stearyl fumarate is usually recommended for use in the range of 0.5–2 %, whereas higher Sodium stearyl fumarate concentrations up to 5 % were used in some studies on minitablets. Due to the hydrophobic nature of the lubricants, adverse effects on tablet tensile strength, disintegration and dissolution have been reported. While Magnesium stearate shows the highest lubrication efficiency, the negative effects on disintegration time, tensile strength and friability are less pronounced for Sodium stearyl fumarate.

Figure 1. Sodium stearyl fumarate was introduced as a suitable lubricant for tableting
Application and Pharmacology
Sodium Stearyl Fumarate is a white or almost white powder that is commonly used in the pharmaceutical industry as a lubricant and glidant in the manufacture of solid dosage forms, such as tablets and capsules. It is a sodium salt of stearyl fumaric acid, which is derived from fumaric acid and stearyl alcohol. Sodium Stearyl Fumarate has good lubricating properties and can help to reduce friction between particles during tablet compression, which can improve tablet quality and prevent sticking to the tablet press. It is also hydrophilic, which means that it can improve the flow and wetting properties of the powder blend, making it easier to process. Lubricants are essential for all solid dosage forms. Lubricants can reduce interparticle friction, facilitate the ejection of the tablet from the die cavity and improve the flow rate of the tablet granulates. Compared with magnesium stearate and talc, Sodium Stearyl Fumarate has several advantages: it is an inert, hydrophilic lubricants for tablets – solves incompatibilities with magnesium stearate, provides superior hardness at equivalent compression forces and less impact on disintegration time; it is semi soluble, hence gives low residue in solution or effervescent preparations. In other words, sodium stearyl fumarate is an excellent substitute for magnesium stearate and talc. The amount of Sodium Stearyl Fumarate used is usually in the range of 0.25 to 3% w/w.
1. Impact of lubrication on key properties of orodispersible minitablets in comparison to conventionally sized orodispersible tablets. Orodispersible minitablets (ODMTs) offer several benefits like easy swallowability, dose flexibility and simple manufacturing through direct compression. In this study, the effect of lubrication on five different co-processed excipients (Ludiflash®, Parteck® ODT, Prosolv® ODT G2, galenIQ™ 721 and SuperTab® 50 ODT) has been studied for orodispersible tablets (ODTs) with 11.28 and 2 mm in diameter. External lubrication was compared with internal lubrication using 0.5 %, 1 % or 2 % magnesium stearate or 1 %, 2 % or 4 % sodium stearyl fumarate. Mechanical strength and disintegration time of the ODTs were evaluated beside the lubrication efficiency. Especially mannitol-based co-processed excipients show strong dependency of the lubricant concentration whereas both ODTs and ODMTs and minitablets with isomalt showed comparable properties for both lubricants and their concentrations. Sodium stearyl fumarate is considered as the preferred lubricant for ODMTs as it showed a higher lubrication efficiency and less negative impact on disintegration time. External lubrication exhibited higher tensile strength for plastic materials, but increased the disintegration time, particularly for ODMTs due to the high specific surface where the lubricant is applied[1].
2. Application of a quality-by-design approach for utilizing sodium stearyl fumarate as a taste-masking agent in dextromethorphan hydrobromide orally disintegrating tablets. Optimizing the utilization of lubricant sodium stearyl fumarate in the preparation of dextromethorphan hydrobromide ODTs with enhanced taste-masking properties. Simple blending of sodium stearyl fumarate with the powder bed would result in taste-masking through physical adsorption of the lubricant particles on the drug particles[2].
Indeed, some resources advised against the use of magnesium stearate in ODTs owing to its hydrophobic nature and tendency to increase the disintegration time of tablets. Sodium stearyl fumarate (SSF), on the other hand, is less hydrophobic, and generally recommended for use in ODTs; it is not sensitive to the blending time factor with regard to its lubricant activity.
Synthesis
Sodium stearyl fumarate is a sodium salt of stearyl fumarate, which is used as an excipient in the pharmaceutical industry to improve the flowability and compressibility of powders. The synthesis of sodium stearyl fumarate can be carried out using the following steps:
1.Synthesis of stearyl fumarate: Stearyl fumarate can be synthesized by reacting fumaric acid with stearyl alcohol in the presence of a catalyst such as sulfuric acid or p-toluenesulfonic acid. The reaction is carried out under reflux conditions, and the product is purified by recrystallization.
2.Neutralization with sodium hydroxide: Once the stearyl fumarate is synthesized, it is neutralized with sodium hydroxide to form the sodium salt of stearyl fumarate. The reaction is carried out in a suitable solvent such as water or ethanol, and the product is purified by filtration or recrystallization. The overall reaction can be represented as follows:
Stearyl alcohol + fumaric acid + NaOH → Sodium stearyl fumarate + Water
The purity and yield of sodium stearyl fumarate can be improved by optimizing the reaction conditions such as the reaction temperature, time, and concentration of reactants. It is important to carry out the synthesis and purification steps under controlled conditions to ensure the quality of the final product.
Toxicity and safety
Sodium Stearyl Fumarate is generally considered safe and is approved for use as a food additive by the FDA. It is also used in other industries, such as the cosmetics industry, as an emulsifier and thickening agent.
1.The Absorption and Metabolism of Orally Administered Tritium Labeled Sodium Stearyl Fumarate in the Rat and Dog. Sodium stearyl fumara: labeled with tritium at carbon atom 1 of the dearyl alcohol moiety was administered by stomach tube to rats and dogs. Examination of excreta and body fluids indicated that in the rat approximately 80% of the dose was absorbed. The major portion of absorbed sodium stearyl fumarate was metabolized within 2 hr following administration, and was completely metabolized in less than 8 hr. Tritium water was the source of the only significant radioactivity found in body fluids. The sodium stearyl fumarate that was not absorbed, approximately 20% of the administered dose, was excreted in the feces as a mixture of stearyl fumarate and stearyl alcohol. When the experiment was repeated with rats which had received 300 mg/kg unlabeled sodium stearyl fumarate daily for 90 days (stressed rats), the absorption and metabolism of sodium stearyl fumarate was indistinguishable from results obtained with control untreated rats. In the dog, approximately 35% of the administered dose of sodium stearyl fumarate was absorbed and rapidly metabolized. Tritium water was the only source of significant radioactivity found in body fluids within 8 hr after administration. Sodium stearyl fumarate not absorbed, ap- proximately 65% of the dose, was excreted un- changed in the feces within the first 24 hr. The metabolism of sodium stearyl fumarate is qualitatively the same in the rat and dog[3].
2. Degradation caused by incompatibility between sodium stearyl fumarate and AZD7986 in the drug product[4]. An incompatibility between sodium stearyl fumarate and a secondary amine have been shown for the first time. The degradant appeared in AZD7986 tablets with sodium stearyl fumarate as one of excipients under humid condition, and was identified as a Michael addition product of API and fumaric acid. Fumaric acid was generated by the hydrolysis of sodium stearyl fumarate in the presence of other excipients and API. The reaction is facilitated by water and by basic conditions. The results from this study should serve as a precautionary note for drug products using sodium stearyl fumarate as an excipient where the API could act as a nucleophile. In such cases, the microenvironment should be optimised to minimize the reaction, by adjusting the pH and incorporating protection from moisture.
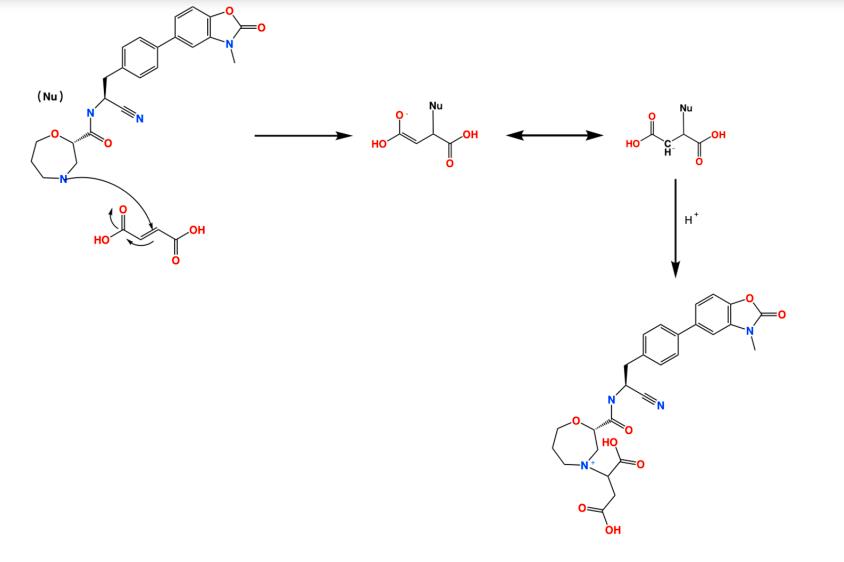
Figure 2 Degradation caused by incompatibility between sodium stearyl fumarate and AZD7986 in the drug product.
Reference
1.
2.
3. Figdor S. K. & Pinson R., "The absorption and metabolism of orally administered tritium labeled sodium stearyl fumarate in the rat and dog," J Agric Food Chem, Vol.18, No.5(1970), pp.872-877.
4.
Related articles And Qustion
See also
Lastest Price from Sodium Stearyl Fumarate manufacturers

US $0.00-0.00/kg2025-07-28
- CAS:
- 4070-80-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT

US $2.00-5.00/kg2025-07-08
- CAS:
- 4070-80-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg